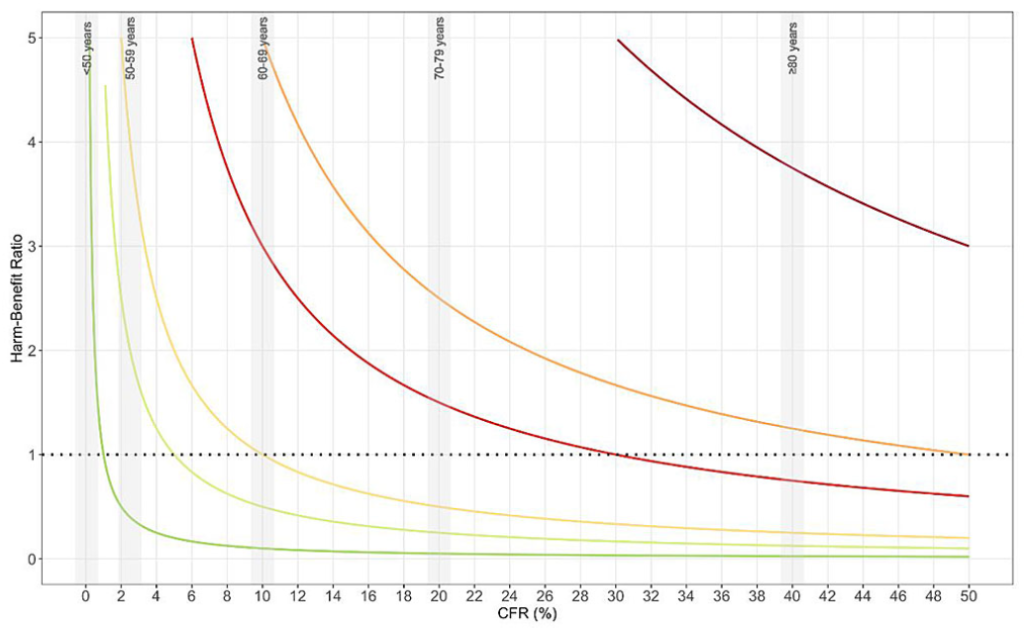
Along with researchers from the University of Basel and Zürich, I have recently written an article about off-label treatments during pandemics: These treatment decisions should reflect the estimated harm-benefit ratio and become, whenever possible, part of a platform trial (many blueprints for future international emergencies are on the way). Contrary to a topical JAMA viewpoint by Dr Califf et al. (Verily Life Sciences, Alphabet), routine healthcare data and learning health systems can play an important role in evaluating potential off-label treatments and safety signals across patient subgroups, but the global research community has to be more careful in communicating observational findings. Last but not least, there is a huge potential for routine healthcare data when used in pragmatic trials.
Applications of routine health(care) data are manifold:
- descriptive studies / monitoring and surveillance
- explorative analyses / hypothesis generation
- risk factor analyses
- predictive modelling
- long-term evaluation of health interventions (effectiveness and safety profile)
- evaluation of the generalizability of trial results and potential subgroup effects / population-based assessments (e.g. CPRD in the UK)
- assessment of broader impact of health interventions
- trial planning (estimated effects, baseline risk, intracluster correlations, etc.)
- trial recruitment (identification of eligible patients)
- pragmatic platform trials
- data source for modelling projects
Califf RM, Hernandez AF, Landray M. Weighing the benefits and risks of proliferating observational treatment assessments: observational cacophony, randomized harmony. JAMA. Published online July 31, 2020. doi:10.1001/jama.2020.13319
Roth JA, Ballouz T, Kouyos RD, Battegay M. Early off-label treatment during pandemics? A dilemma. Swiss Med Wkly. 2020 May 11;150:w20281. doi: 10.4414/smw.2020.20281. eCollection 2020 May 4.